- Home
- Services
- Products
- Technology
Fluorescent Dye-Labels FRET Substrates Phosphorylation Isotope Labeling Stapled Peptides Disulfide Bridges Amide Cyclization Peptide Libraries Glycosylation Lipopeptides PEGylation Peptoids Unnatural Amino Acids MAPs Linker Spacer Cell-penetrating peptides Chelating Peptides Insoluble Peptide Purification Long Peptide Synthesis N-terminal modifications C-terminal modifications
- Customer Support
- Technical Support
- News
- Contact Us
- | 中文版 |










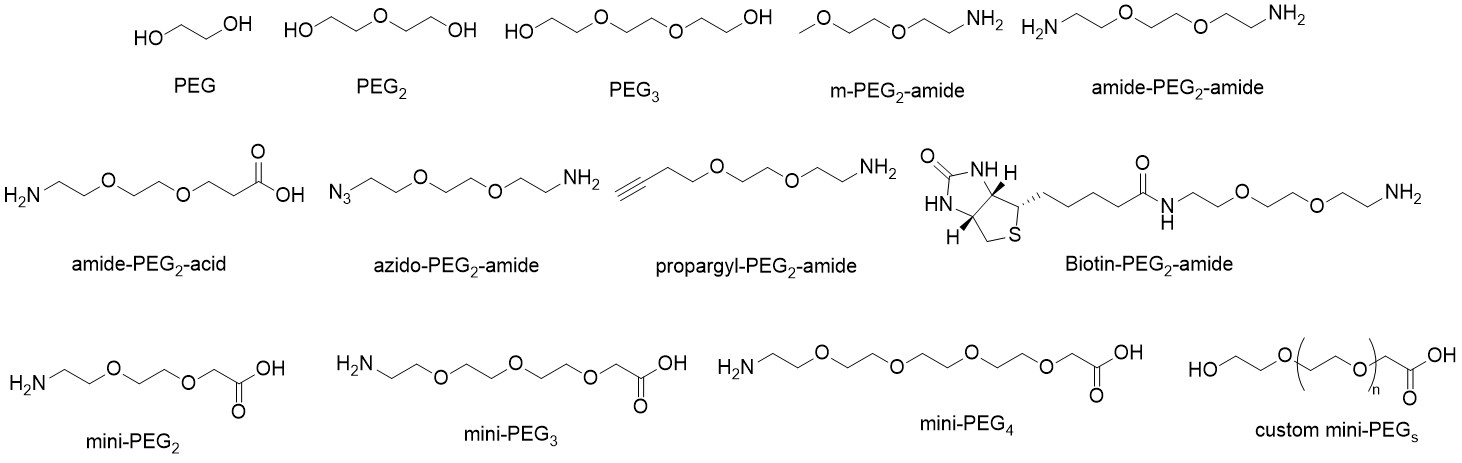
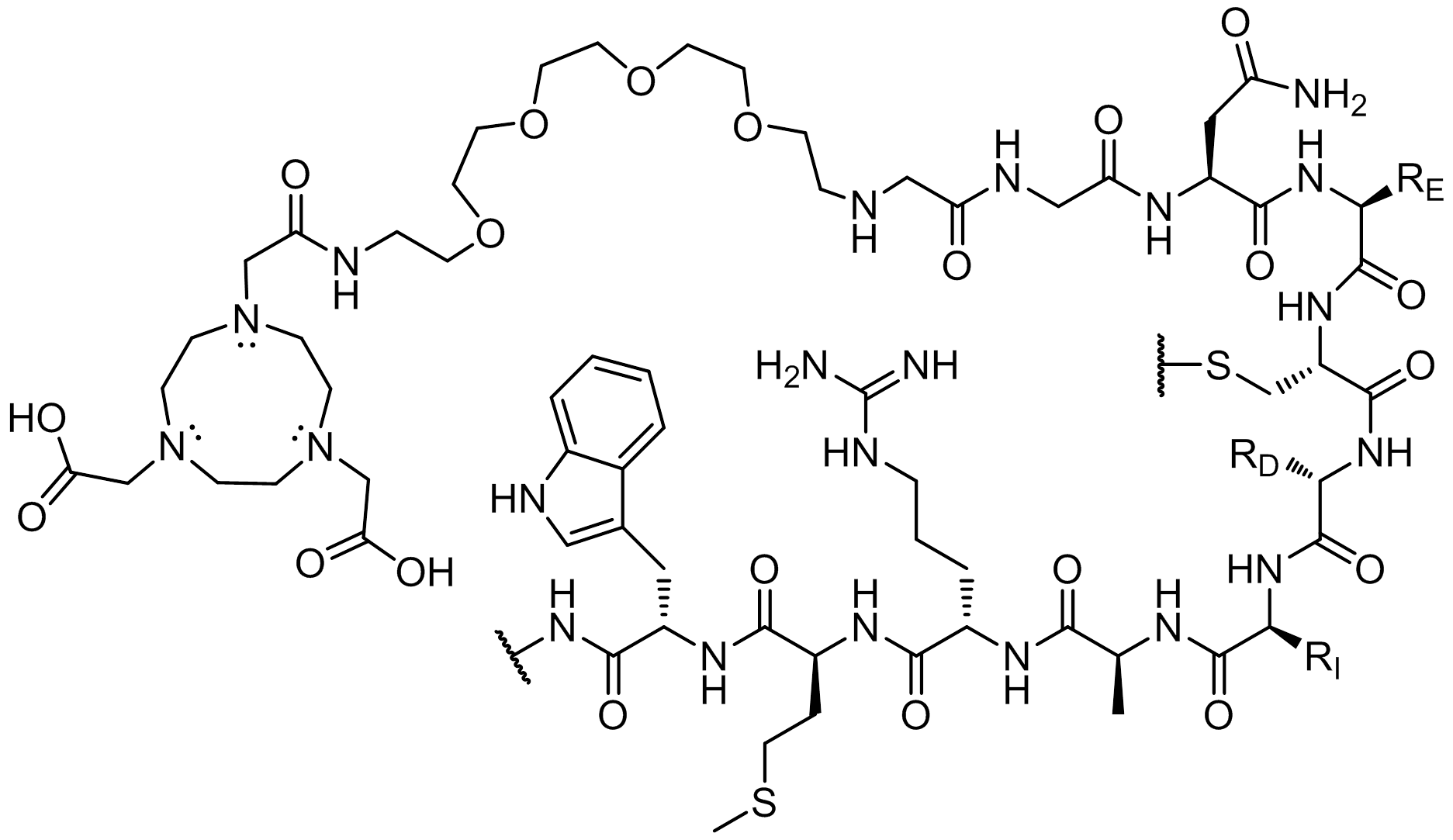 The resultant sequence, NOTA-PEG4- GGNECDIARMWEWECFERK-NH2, was then cross-linked with 5-fluoro-2,4-dinitrobenzene to form a covalent bond with lysine-19 (L19K-FDNB). This modification increases binding affinity because it enables the peptide probe to irreversibly bind to VEGF by a covalent attachment to a site inside the binding pocket of the protein. The inert and flexible characteristic of the PEG linker provides distance between the peptide cargo carrier (e.g., NOTA) for optimal binding to the receptor.
The resultant sequence, NOTA-PEG4- GGNECDIARMWEWECFERK-NH2, was then cross-linked with 5-fluoro-2,4-dinitrobenzene to form a covalent bond with lysine-19 (L19K-FDNB). This modification increases binding affinity because it enables the peptide probe to irreversibly bind to VEGF by a covalent attachment to a site inside the binding pocket of the protein. The inert and flexible characteristic of the PEG linker provides distance between the peptide cargo carrier (e.g., NOTA) for optimal binding to the receptor.




 Contact us by We-chat.
Contact us by We-chat.



