- Home
- Services
- Products
- Technology
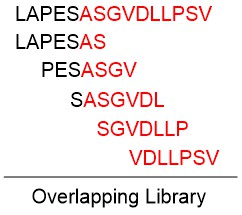
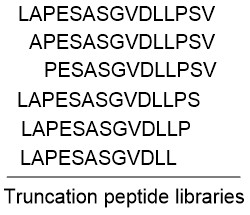
Fluorescent Dye-Labels FRET Substrates Phosphorylation Isotope Labeling Stapled Peptides Disulfide Bridges Amide Cyclization Peptide Libraries Glycosylation Lipopeptides PEGylation Peptoids Unnatural Amino Acids MAPs Linker Spacer Cell-penetrating peptides Chelating Peptides Insoluble Peptide Purification Long Peptide Synthesis N-terminal modifications C-terminal modifications
- Customer Support
- Technical Support
- News
- Contact Us
- | 中文版 |


















 Contact us by We-chat.
Contact us by We-chat.



