Peptide drugs are a rapidly growing class of therapeutics. However, the peptide drug discovery has been hampered by its inherent characteristics: low stability due to susceptibility to enzymatic digestion, low target specificity because of high conformational flexibility, low hydrophobicity and the lack of specific transportation systems. In order to overcome these disadvantages of peptide, HongTide has developed an innovative peptoid synthesis service to meet the increasing needs in drug target discovery and lead structure discovery research.
Peptoids, or poly-N-substituted glycines, are a class of peptidomimetics whose side chains are appended to the nitrogen atom of the peptide backbone rather than to the α-carbons (as they are in amino acids). In native peptides, R group represents 20 different substitutions for specific amino acids, while in peptoids, the selection of R groups can be much wider and potentially unlimited.
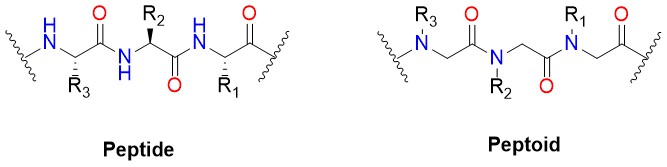
Key Features (Peptoid VS. Peptide)
-
More stable:
Peptoids are less susceptible to degradation in vivo than peptides;
-
More choices:
Peptoids are well suited for combinatorial approaches to drug discovery because large libraries can be synthesized easily from readily available primary amines;
-
More cost-effective and time-efficient:
Major advances in screening methodology have allowed peptoid libraries of hundreds of thousands of compounds to be mined inexpensively and quickly for highly specific protein-binding;
-
Higher market potential:
The features of peptoids make them a class of pharmacological agents with great potential.
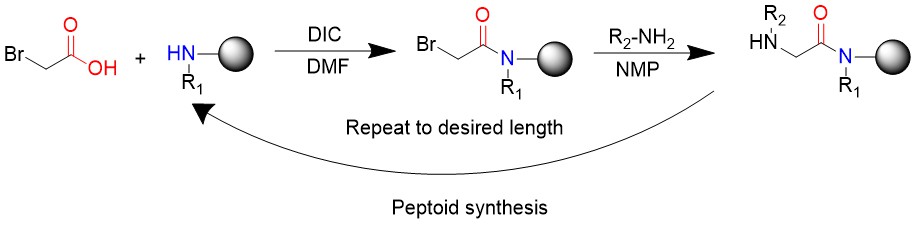
Peptoids Synthesis Introduction
Submonomer method, developed by Ron Zuckermann, is the widely used method to synthesize peptoids. Two steps will be take to complete the reaction: acylation and displacement. Comman reaction process to synthesize peptoids is:
Current Available Peptoid Side Chain
Abbreviation
NAla
NAbu
Nnpa
NVal
Nnba
NIle
NLeu
NPhe
Npea
Nmba
NMet
Nmpa
NLys
Nmpe
Ndmb
Nffa
Npip
Nbsa
Ntbu
Ndip
Side Chain Name
Methylamine
Ethylamine
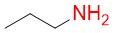
n-propylamine
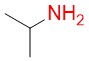
Isopropylamine
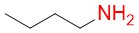
n-butylamine
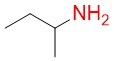
Sec-butylamine
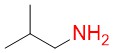
Isobutylamine
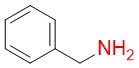
Benzylamine
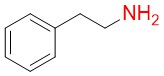
β-phenylethylamine
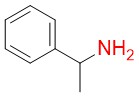
α-methylbenzylamine
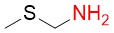
2-(methylthio)ethylamine
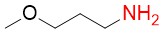
3-Methoxypropylamine
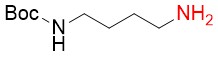
N-Boc-1,4-Diaminobutane
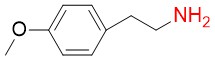
4-methoxyphenethylamine
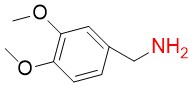
3,4-Dimethoxybenzylamine
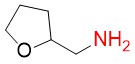
(S)-(+)-Tetrahydrofurfurylamine
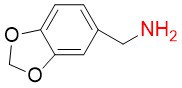
Piperonylamine
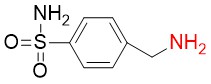
4-(2-Aminoethyl)benzenesulfonamide
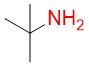
Tert-butylamine
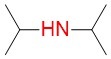
diisopropylamine


















 Contact us by We-chat.
Contact us by We-chat.



