-
Kinase Activity Assays
Kinase activity can be measured in vitro by incubating an immunoprecipitated kinase with a specific substrate in the presence of ATP. The phosphorylated substrate can then be measured using colorimetric, radioactive, or fluorometric reporter systems.
-
The Development of Phospho-Specific Antibodies
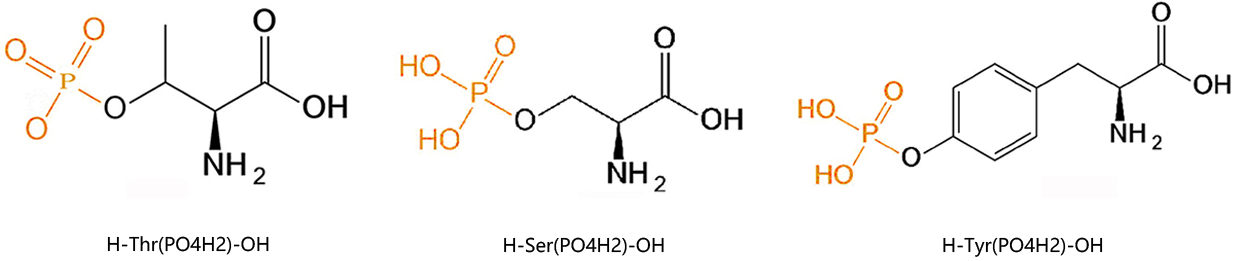
Many scientists develop phosphorylation-specific antibodies to analyze protein phosphorylation. First, phospho-specific peptides that contain the amino acid sequence surrounding the phosphorylation site of interest within the target protein was synthesized, which was then conjugated to KLH for immunization. The immune sera were then applied to a peptide affinity column to yield a highly specific antibody. Successful detection of the phosphorylated target protein depends on the affinity of the antibody for the phosphoprotein of interest and its specificity.
-
Western Blot
Many phospho-specific antibodies are highly sensitive, which means that they can easily detect phosphorylated proteins in biological samples. Chemiluminescent and colorimetric detection methods are both conventional, which are generally used in conjunction with molecular weight markers to provide information regarding the molecular mass of the target protein.
-
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISAs generally provide indirect measurements kinase activity and are more quantitative than western blotting. The results of phospho-specific ELISAs can by quantified easily using calibrated standards. Two antibodies that are specific for the target protein are commonly used together in a sandwich format to yield high specificity. In addition, the microplate-based format of ELISAs allows high throughput analysis using smaller sample volumes, as well as the detection of low-abundance proteins.
-
Cell-Based ELISA
The analysis of protein phosphorylation in intact cells might represent the activity of specific signaling networks more accurately. Generally, phospho-specific antibodies are used to assess the phosphorylation status of target proteins using fluorometric or colorimetric detection systems.
-
Intracellular Flow Cytometry and ICC/IHC
Flow cytometry is an advantageous method for analyzing protein phosphorylation because it allows rapid and quantitative analyses at the single cell level. Cells are usually stimulated, and fixed with formaldehyde or paraformaldehyde to cross-link and stabilize the phospho-proteins before analysis. The fixed cells are then permeabilized to allow the phospho-specific antibodies to enter the cells.
-
Mass Spectrometry
Mass spectrometry (MS) is a useful tool for identifying specific phospho-proteins and phosphopeptides and for sequencing the specific phosphorylated residue. MS affords excellent sensitivity and resolution, which allows users to identify individual proteins or peptides. Although the signals from phosphopeptides are generally weaker than those from non-phosphorylated peptides, novel technologies have been developed that would enable the MS signal to be enriched. Examples of such enrichment strategies include immobilized metal affinity chromatography, phosphospecific antibody enrichment, chemical modification-based methods, and replacing a phosphate group with biotinylated moieties.
-
Multi-Analyte Profiling
Multi-analyte profiling uses phospho-specific antibodies followed by microplate- or membrane-based detection. These assays provide large amounts of data and require a minimal sample volume. However, they are less sensitive than conventional methods because of potential antibody cross-reactivity.














 Contact us by We-chat.
Contact us by We-chat.



