Stapled peptides are peptides locked into their bioactive alpha-helical conformation through site-specific introduction of an all-hydrocarbon staple. Peptide stapling was developed to overcome the limitations of two broad classes of therapeutic agents (small molecules and protein biologics) in targeting intracellular protein-protein interactions. Small molecules only work on proteins with a specific feature on their surfaces and most protein biologics do not penetrate into cells. Because stapled peptides are locked into a stabilized α-helical structure (the most common element of protein secondary structures), they can easily penetrate cells. As a rapidly emerging class of next-generation drugs, stapled peptides are expected to combine the synthetic manipulability and cell-penetrating ability of small molecules with the three-dimensionality and versatile target recognition ability of biologics.
HongTide has extensively developed stapled peptide structures and is the company of choice to manufacture your stapled peptide requirements. Our technical consultants would be happy to discuss your structural design needs with you at any time.
Stapled Peptides Synthesis Introduction
In order to synthesize stapled peptides, we usually introduce two alpha-4-n-pentenylalanine (S5) residues into a peptide strand enables ring-closure metathesis to create a single stapled peptide. When n = 3 (i.e. with 3 amino acids between the S5 residues) the staple type is known as an i, i + 4. Grubbs catalyst’s are routinely used in olefin metathesis to incorporate hydrocarbon staples into peptides.
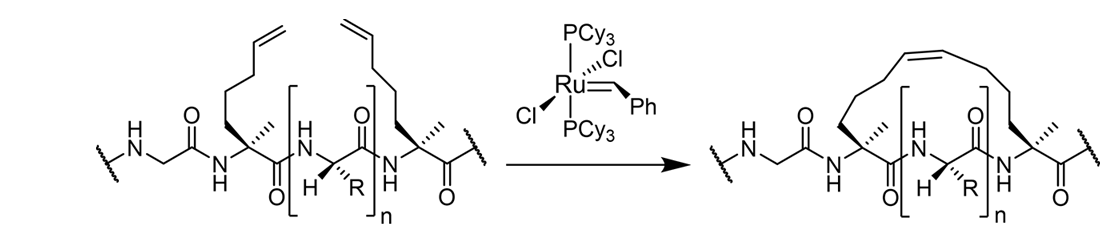 Approximately one turn of the helix would be i and i+4 and two turns of the helix would be i, i+7. Two different types of hydrocarbon staples are shown in the figure below, demonstrating the creation of a stabilized α-helix in a peptide.
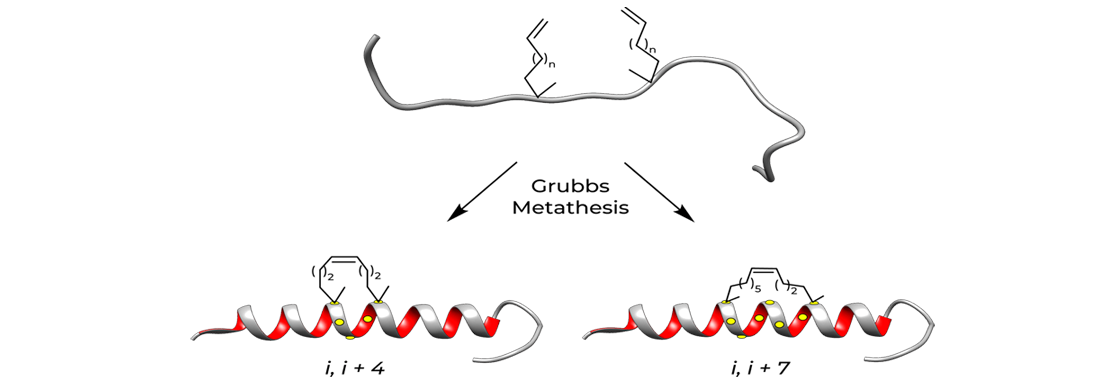
Approximately one turn of the helix would be i and i+4 and two turns of the helix would be i, i+7. Two different types of hydrocarbon staples are shown in the figure below, demonstrating the creation of a stabilized α-helix in a peptide.
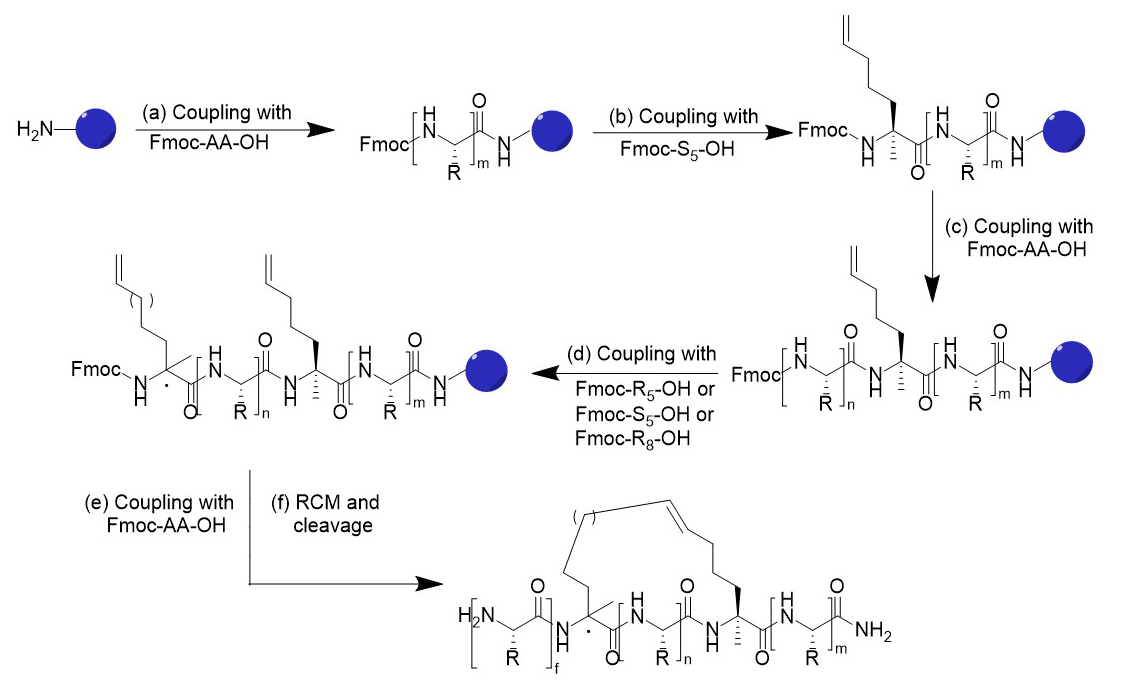
 Synthesis Route of Stapled Peptide.
Synthesis Route of Stapled Peptide.
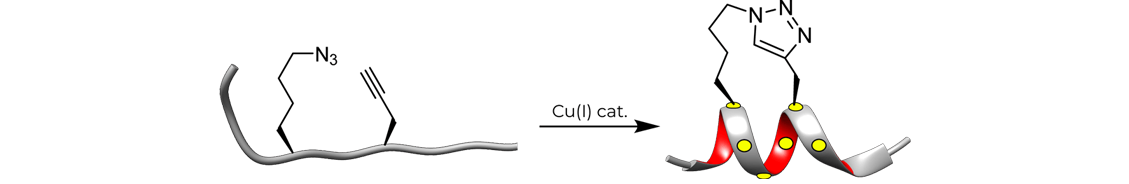
Stapled Peptides by Click Chemistry
Combined with the ease of synthesis of the necessary unnatural amino acids, "click" chemistry(Copper-catalyzed Huisgen 1,3-dipolar cycloaddition reaction) can be used for acile synthesis of triazole-stapled peptides because of its high efficiency and mild conditions. For example, a combination of L- Nle (εN3) and D-Pra (D-propargylalanine) substituted at the i and i+4 positions can be used for the generation of single triazole-stapled peptides.
HongTide have succeeded in synthesizing many stapled peptides by click chemistry.
Feature
Extensive experience on stapled peptides synthesis.
Strict quality inspection standard, HPLC and MS will be provided.
Modification can be made to stapled peptides,such as D-Biotin, fluorescent dyes etc.
Purity up to 95%.
Stapled Peptides in Drug Design
Better target affinity (5 to 5,000-fold increase);
Increased proteolytic resistance and serum half- life;
Cell penetration through endocytic vesicle trafficking;
Targeting of either extracellular or intracellular proteins;
Disruption of protein-protein interactions;
Non-immunogenicity.










 Approximately one turn of the helix would be i and i+4 and two turns of the helix would be i, i+7. Two different types of hydrocarbon staples are shown in the figure below, demonstrating the creation of a stabilized α-helix in a peptide.
Approximately one turn of the helix would be i and i+4 and two turns of the helix would be i, i+7. Two different types of hydrocarbon staples are shown in the figure below, demonstrating the creation of a stabilized α-helix in a peptide.
 Synthesis Route of Stapled Peptide.
Synthesis Route of Stapled Peptide.






 Contact us by We-chat.
Contact us by We-chat.



